Research projects
Enzymes can catalyze a large number of chemical reactions, limiting the use of organic solvents, and operating under mild conditions (temperature, …). In Enzymology and Glycochemistry group, two enzymes families are used, involved in vivo in synthesis (Glycosyltransferases) or breaking (Glycosides Hydrolases) of carbohydrates.
Our methodological approach, bridging fundamental to applicative aspects can be decomposed in 3 parts:

1. Biocatalysts identification & characterization.
Using a panel of techniques ranging combining molecular biology (genomic analysis, cloning, ...), protein biochemistry (expression, purification...), enzymology (biocatalysus, kinetics, ...), and structural biology (molecular modeling, protein x-ray crystallography, ...), candidates enzymes are identified, produced and characterized. Their selection is led by genomic annotations data, desired characteristics, as well as potential substrate selectivity (eg. acting on rare sugars).
3 recent examples :
| α-L-Rhamnosidase (DtRha) Guillotin et al, 2019, ACS Omega | |
|
DtRha can selectively hydrolyze rhamnosides moeties in natural flavonoids, paving the way to intermediate metabolites synthesis. |
|
| β-D-Galactofuranosidase (Galf-ase) – Seničar et al, 2019, Carb Res. | |
|
Galf-ase is the first cloned galactofuranosidase that can specifically hydrolize galactofuranose. This rare sugar is found as a constituent of the cell wall of many pathogenic microorganisms. |
 |
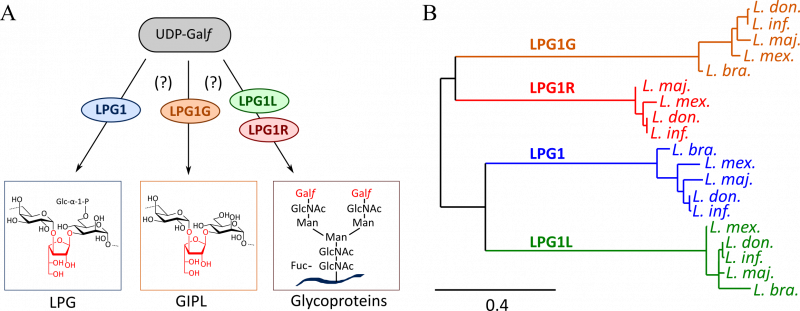
| Galactofuranosyltransférases LPG1 – Ati et al, 2018, Sci Rep. | |
|
4 galactofuranosyltransferases LPG1 from Leishmania major were produced and demonstrated an original substrate specificity, paving the way for innovative biocatalyzed synthesis. |
 |
2. Protein Engineering
Genetic engineering (directed mutagenesis, ...) can tailor the peptidic sequence of enzymes. In Enzymology & Glycobiochemistry group, we use these techniques aiming at 2 goals : understanding of the mechanism of these enzymes at a molecular level, and modulating the activity of the biocatalysts to improve their efficiency or their selectivity..
3 recent examples:
| S-Glycosyltransferase UGT74B1 – Lafite et al., 2019, Mol Cat | |
|
A. thaliana UGT74B1 activity is correlated to the acidity of the reactive thiol. Site-directed mutagenesis enable to identify catalytic residues involved in this original reaction mechanism. |
|
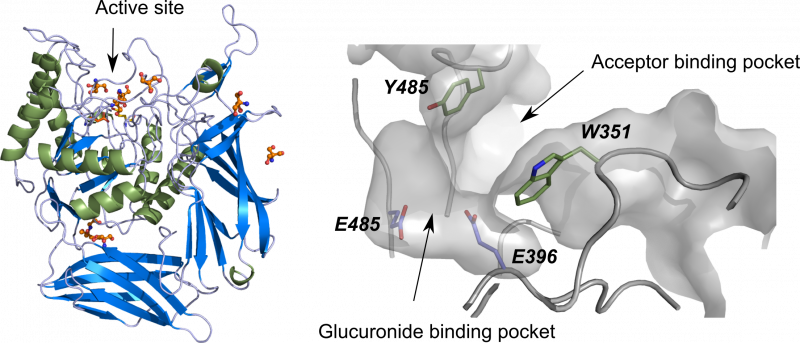
| β-D-Glucuronidase DtGlcA – Kurdziel et al, 2020, Org. Biomol. Chem. | |
|
Mutagenesis of a catalytic residue can inactivate the hydrolytic activity of glycoside hydrolases. In several cases, this mutation enables to turn the activity from hydrolysis to thioligation, that can be used to synthesize S-glycosides. This methodology was successfully used in the group, including DtGlcA. |
  |
| β-D-Glycosidase DtGly– Guillotin et al, 2019, Catalysts | |
|
Thioimidates as original sugar donors were studied in the case of DtGly, and mutagenesis enabled the identification of the molecular mechanism of the enzyme. |
 |
3. An application: preparation of O-glycosides as cosmetics preservatives (Guillotin et al., 2017, Pure and Applied Chem.).
An enzyme cloned from D. thermophilum was used as a biocatalysts to synthesize a series of glycosylglycerol derivatives. Some of these compounds were found to exhibit an antimicrobial activity towards Aspergillus strains under a microchallenge assay.











